Prevention of Progression to Cirrhosis in Hepatitis C With Fibrosis
Effectiveness and Cost Effectiveness of Sequential Therapy With New Direct-acting Anti-virals
Aliment Pharmacol Ther. 2016;44(8):866-876.
Aim To identify the cost-effective treatment for patients with HCV infection with F3 liver fibrosis who are at high risk of progression to cirrhosis.
Methods A decision-analytic Markov model compared the health benefits and costs of all currently licensed treatments as single treatments and in sequential therapy of up to three lines. Costs were expressed in pound sterling from the perspective of the UK National Health Service. Health benefits were expressed in quality-adjusted life years.
Results Treatment before progression to cirrhosis always offers the most health benefits for the least costs. Sequential therapy with multiple treatment lines cures over 89% of patients across all HCV genotypes while ensuring a cost-effective use of resources. Cost-effective regimes for HCV genotype 1 patients include first-line oral therapy with sofosbuvir–ledipasvir while peginterferon continues to have a role in other genotypes.
Conclusions The cost-effective treatment for HCV can be established using decision analytic modelling comparing single and sequential therapies. Sequential therapy with DAAs is effective and cost-effective in HCV patients with F3 fibrosis. This information is of significant benefit to health care providers with budget limitations and provides a sound scientific basis for drug treatment choices.
Introduction
The new direct-acting anti-virals (DAAs) represent a step-change in the treatment prospects of the 185 million people around the world with chronic hepatitis C.[1] Simeprevir (SMV), sofosbuvir (SOF), sofosbuvir–ledipasvir (SOF–LED), daclatasvir (DCV) and ombitasvir–paritaprevir–ritonavir with or without dasabuvir (2D/3D) offer high cure rates and fewer adverse effects than standard interferon-based treatments.[2] However, their high prices raise issues regarding their affordability to health care systems and patients.[3]
From an economic perspective, the new DAAs are cost-effective if their health benefits exceed the opportunity cost. The opportunity cost is the health benefit forgone by other patients if interventions are no longer funded to release the resources to fund the new DAAs. Health benefits associated with extension of survival and/or improved health-related quality of life can be summarised as quality-adjusted life years (QALYs). In the UK, the National Institute for Health and Care Excellence (NICE) has appraised each new DAA and has recommended some as cost-effective options.[4–8] When deciding on the cost-effectiveness of new treatments, NICE compares their incremental cost-effectiveness ratio (ICER) to a cost-effectiveness threshold of £20 000–£30 000 per QALY.[9] The ICER describes the additional cost per QALY gained with the new treatment. The cost-effectiveness threshold represents the opportunity cost of programmes that could be displaced by the introduction of new, more costly, interventions.[9] Hence, treatments with ICERs below the threshold are cost-effective because their health benefits exceed their health opportunity costs. Recent empirical evidence suggests that opportunity costs of additional expenditure are as much as 1 QALY per £13 000 additional costs, although the discrepancy between this evidence and NICE's thresholds may partly reflect additional factors that the Institute considers relevant to its decisions for some products.[10]
The health benefits and opportunity costs of the new DAAs can only be assessed accurately if the clinical pathway is appropriately modelled and all the alternative ways in which treatments can be used are compared. In the case of chronic hepatitis C, this means including not only all of the new DAAs but also the older interferon-based treatments and the possibility of treatment at later disease stages. It also means including sequential therapy, whereby patients who fail therapy are retreated, as seen in clinical practice. The cost-effectiveness analyses used to inform NICE appraisals,[4–8] as well as other published cost-effectiveness studies,[11–19] have not done this. Patients were assumed not to be re-treated if they failed therapy and did not have the possibility of treatment at more severe disease stages. Furthermore, due to the timing of the NICE appraisals relative to the licensing of the DAAs, these new treatments have not all been directly compared.
This study addresses these evidence gaps by comparing the health benefits and opportunity costs of all relevant treatment pathways, from the least intensive option of do nothing and treat when patients develop cirrhosis ('watchful waiting'), to treatment with established or new treatments in up to three lines of sequential therapy. The objectives are to establish whether patients who are at high risk of progression to cirrhosis on the basis of having hepatic fibrosis at a significant stage (METAVIR F3) should be treated before progressing to cirrhosis, and to assess how many lines of treatment and which of these treatments should the patients receive.
Methods
Overview
The model identifies the cost-effective treatment strategy as the strategy which achieves the greatest net health. Net health is calculated as the difference between lifetime QALYs generated for hepatitis C patients and the associated lifetime costs converted to health losses within the wider National Health Service (NHS) using the cost-effectiveness threshold of £20 000 per QALY gained.[9,20] Costs are expressed in UK pound sterling at a 2014 price base from the perspective of the UK NHS. Both costs and QALYs are discounted at 3.5% per annum.[9] Further details on the methods are presented in the Data S1, available as supporting information.
Population and Subgroups
The population of interest is patients at the METAVIR F3 stage, who have advanced fibrosis (hereafter referred to as F3) with hepatitis C virus (HCV) genotypes 1–4. The focus is patients at the F3 stage because these patients are most at risk of progression to cirrhosis. The NHS currently uses fibrosis stage as a basis for determining eligibility for treatment, only recommending routine provision of the new DAAs for patients with cirrhosis and decompensated cirrhosis.[21] Given that F3 patients are the most likely to progress to cirrhosis and further complications, establishing the optimum treatment pathway for this group is a priority.
The population characteristics for patients entering the model are obtained from the UK Trent HCV cohort, from the group of patients with severe fibrosis and no history of decompensation (N = 131).[22] Prognosis, effectiveness of treatment and the marketing authorisations for the different regimens vary by HCV genotype, prior treatment experience and eligibility for interferon treatment. Therefore, the patient population is considered in 14 subgroups defined by HCV genotype, prior treatment experience to peginterferon or protease inhibitor-based therapy (treatment naïve, treatment experienced to peginterferon, treatment-experienced to protease inhibitors and peginterferon) and eligibility for interferon treatment (interferon-eligible and interferon-ineligible or intolerant).
Treatment Strategies
Eight treatments are considered: pegylated interferon alfa-2a with ribavirin (RBV; PR, pegylated interferon with RBV),[23] SMV with PR (SMV + PR),[24] SOF with PR (SOF + PR) or with RBV alone (SOF + RBV),[25] DCV with PR (DCV + PR) or with sofosbuvir (SOF + DCV),[26] SOF + LED with or without RBV (SOF + LED ± RBV),[27] and 2D/3D with or without RBV (2D/3D ± RBV),[28] within their product licenses, in addition to watchful waiting. These treatments can be used alone or as sequential therapies. Sequential therapies can comprise two or three lines of treatments. Second-line treatment is only administered to those who fail first-line treatment and third-line treatment is only administered to those who fail second-line treatment. All possible sequential therapies are evaluated apart from sequences that repeat treatments, use PR-containing regimens three times or use PR alone in patients who failed PR in combination with another drug. As a result, the model compares 633 treatment strategies across the 14 subgroups.
The watchful waiting strategy consists of monitoring the patient but withholding treatment until progression to cirrhosis. Treatment in cirrhotic patients follows NHS England's commissioning policy.[21] This consists of SMV + PR, SOF + PR, 2D/3D ± RBV or SOF + DCV ± RBV depending on HCV genotype and eligibility for treatment with interferon. In the model, treatment upon progression to cirrhosis is modelled as a composite strategy over a sequential therapy composed of two treatment lines. Infected patients who progress to decompensated cirrhosis receive one line of treatment. Also modelled as a composite treatment strategy, treatment at decompensated cirrhosis consists of the treatments used in the NHS Early Access Scheme for patients at this stage (SOF + LED ± RBV in HCV genotypes 1, 3 and 4, SOF + DCV + RBV in HCV genotype 3).[21]
The treatments 3D + RBV and 3D for subtypes G1a and G1b are combined as a composite treatment for HCV genotype 1 given their similar effectiveness.[6] The older protease inhibitors, telaprevir and boceprevir, have not been included because they are less effective and more costly than SMV,[4] and their future availability is unclear.[29] SOF + SMV has not been included because, at the time of this analysis, it had not been appraised by NICE and no systematic review was available on its effectiveness.
Table 1 shows the possible components of the treatment strategies considered in each subgroup. For example, in the HCV genotype 2 treatment-naïve, interferon-eligible subgroup, the possible treatments are PR and SOF + RBV. As a result, there are five possible treatment strategies: (i) PR alone; (ii) SOF + RBV alone; (iii) PR followed by SOF + RBV in patients who failed PR; (iv) SOF + RBV followed by PR in patients who failed SOF + RBV, and (v) watchful waiting (no treatment until progression to cirrhosis).
Modelling Approach
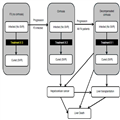
The treatment goal in chronic hepatitis C is to achieve sustained virological response (SVR), which is widely regarded as cure from the infection.[30] Figure 1 depicts the model structure. Patients at the F3 stage can be treated up to three times, depending on the treatment strategy under evaluation and may or may not achieve SVR following each treatment. Patients who progress to cirrhosis or decompensated cirrhosis are treated as per NHS England's commissioning policy.[21] Patients with cirrhosis are at risk of progression to decompensated cirrhosis and hepatocellular cancer, which in turn are associated with greater mortality risk. Patients at the decompensated cirrhosis or hepatocellular cancer states may have liver transplantation, which improves their prognosis and health-related quality of life. Patients with decompensated cirrhosis are at risk of developing hepatocellular cancer. SVR in the F3 state is assumed to halt disease progression, while in cirrhosis and in decompensated cirrhosis it is assumed to reduce the speed of disease progression. Additionally, SVR is assumed to improve health-related quality of life.
Figure 1.
Model diagram. The squares represent health states; the arrows represent the flows allowed in the model. Patients are at risk of death from all causes from all model states (not shown). All patients start in the F3 state with hepatitis C infection. Patients can be retreated up to three times at F3. Patients who achieve SVR are no longer at risk of progression and experience the mortality risk of the general population. Patients who do not achieve SVR can progress to cirrhosis. Upon progression to cirrhosis, patients can be treated up to two times. Patients with cirrhosis or decompensated cirrhosis who have achieved SVR can progress but at a slower rate than those without SVR.
Model Inputs
The model inputs were mostly obtained from the cost-effectiveness analyses informing the NICE technology appraisals on the new DAAs,[4–8] complemented with targeted literature searches. The NICE appraisal process requires drug manufacturers to present a systematic review on the effectiveness of the drug in question; which is then critically appraised by an independent academic group. This process is scrutinised by the NICE Appraisal Committee, commentators and consultees. The NICE Appraisal Committee uses this information to decide on the effectiveness and cost-effectiveness of the new drug to the NHS. Given this thorough review process, the parameters used to inform NICE decisions represent the best available evidence and hence are used within the model.
Treatment-specific Parameters
Table 2 shows the SVR rates used in the model for F3 patients (for details see Data S1 pages 5–12). These correspond to the SVR rates in noncirrhotic patients used in the NICE appraisals. Data for PR are re-extracted from the source publications used in the NICE appraisals due to inconsistencies in the selection of subgroup data and type and dosage of peginterferon. SVR rates are generalised across some subgroups as conducted in the NICE appraisals, namely from interferon-eligible to interferon-ineligible and from HCV genotype 1–4 (e.g. SOF + LED). Furthermore, the SVR rates in treatment-experienced patients to PR are generalised to treatment-experienced patients to new DAAs and to treatment-experienced patients to two treatment lines. In the model, the SVR rates are converted into log odds of SVR response and a random deviate of mean zero and variance 0.015 was added to reflect the possible bias from conducting naïve comparisons of individual study groups.[31] SVR rates from randomised comparisons were unaffected (see Data S1 pages 13–14 for details).
The SVR rates at the cirrhotic stage are obtained from the cirrhotic subgroup presented in the NICE appraisals. For decompensated cirrhosis, the SVR rates are obtained from the results of the NHS Early Access Scheme.[31] The SVR rate of treatments at cirrhosis stage is between 93% and 99% depending on subgroup; for decompensated cirrhosis varies between 65% and 84% (see Data S1 pages 24–26 for details).
During the treatment period, the model captures the adverse effects of treatment as a reduction in health-related quality of life. There is limited evidence on the existence of long-term adverse consequences from PR and no evidence is available yet for the new DAAs.
Table 3 shows the treatment prices, which are the publicly available list prices in England.[32] As prices can incorporate confidential discounts negotiated locally, an Excel tool is available to download (see Data S2) to recalculate the cost-effectiveness results from a set of user-defined prices.
Common Parameters
Parameters that were common across treatments (transition probabilities, health-state costs, monitoring costs and health-related quality of life weights) are obtained from the publications used in the previous NICE appraisals.[33–39]
The improvement in health-related quality of life from achieving SVR varied across NICE appraisals[4,7,8] and was not specific to patients with advanced disease. In F3 and cirrhotic patients, this input is obtained from a study reporting the improvement in health-related quality of life specifically in patients with METAVIR F3–F4.[40] The health-related quality of life improvement in patients who achieved SVR at decompensated cirrhosis is estimated by assuming that patients would experience health-related quality of life at the midpoint between that of patients with cirrhosis and those with decompensated cirrhosis. This was also the approach taken to obtain the cost of decompensated cirrhosis with SVR.
Analytic Methods
The base-case results reflect the average costs and QALYs across 5000 Monte Carlo simulations representing uncertainty in the parameters estimated from the available evidence. The cost-effective strategy and the probability that this strategy is cost-effective are evaluated at a threshold of £20 000 per QALY. The probability of cost-effectiveness represents the likelihood that a specific strategy is the best policy decision.[9]
The evaluation in treatment-experienced patients was conducted by comparing the subset of treatment sequences using PR or protease inhibitor-based therapy as a first-line treatment.
Scenario Analysis
Scenario analyses explore the sensitivity of the results to key assumptions and parameter inputs, specifically: data used to estimate the rate of progression of liver disease, the effect of achieving SVR on progression and health-related quality of life, the impact of treatment at cirrhosis and decompensated cirrhosis, age at treatment initiation and adherence to PR.
Results
Base-case
Table 4 presents the base-case results for treatment-naïve patients. Only the strategy with the greatest net health (i.e. the cost-effective strategy) using NICE's threshold is shown. Full results for all the strategies are presented in the Data S1 pages 44–65. In general, the cost-effective strategies include two or three lines of treatment and result in approximately 90% of patients achieving SVR without progression to cirrhosis. Across all subgroups, the cost-effective strategies ensure that a maximum of 10% of patients progress to cirrhosis and a maximum of 4% to decompensated cirrhosis. Watchful waiting (i.e. waiting until progression to cirrhosis to offer treatment) is clearly not cost-effective as, in all subgroups, it is more costly and less effective than the cost-effective strategy.
The results indicate that patients with HCV genotype 1 should receive SOF + LED over 8 weeks as first-line treatment and 3D ± RBV as second-line treatment; the probability that this sequential therapy is cost-effective is 0.97 (not shown in Table 4 ). Third-line treatment should be SOF + PR in those who are eligible for interferon and SOF + DCV in those who are not.
The cost-effective strategy for HCV genotype 2 is also subject to minimal uncertainty. The probability that a strategy with PR as first-line treatment and SOF + RBV as second-line treatment is cost-effective in those eligible for interferon is 0.95 SOF + RBV is the cost-effective treatment for patients ineligible or intolerant to interferon (probability = 0.98).
In patients with HCV genotype 3 eligible for interferon treatment, the cost-effective strategy is PR as first-line treatment, SOF + PR as second-line treatment and SOF + LED + RBV as third-line treatment. There is a very high probability (0.97; not shown in Table 4 ) that PR should be used in the first line treatment, however, there is uncertainty regarding the choice of second- and third-line treatment (with a 0.49 probability that SOF + PR should be used before SOF + LED + RBV and a 0.45 probability that it should be used after). For interferon-ineligible or -intolerant patients, the cost-effective strategy is SOF + LED + RBV as first-line, SOF+DCV as second-line and SOF+RBV as third-line treatment (probability = 0.68).
In patients with HCV genotype 4 who are interferon eligible, the cost-effective strategy is PR as first-line, 2D+RBV as second-line and SOF+LED as third-line treatment (probability = 0.60). Overall, the probability that PR is cost-effective as the first-line treatment is 0.63 (not shown in Table 4 ). In interferon-ineligible or -intolerant patients, the cost-effective strategy is 2D + RBV as first-line, SOF + LED as second-line and SOF + DCV as third-line treatment (probability = 0.71).
Treatment-experienced Patients
In HCV genotype 1 treatment-experienced patients, the cost-effective first-line treatment is 3D ± RBV and second-line treatment is SOF + LED (probability = 0.96). In HCV genotype 2 treatment-experienced patients, the cost-effective first-line strategy is SOF+RBV (probability = 0.95). For HCV genotype 3, the cost-effective first-line strategy is SOF+PR and second-line strategy is SOF + LED + RBV (probability = 0.48). For HCV genotype 4, the cost-effective first-line strategy is 2D + RBV and second-line strategy is SOF + LED (probability = 0.97).
Scenario Analysis
The cost-effective strategies are generally robust to the scenarios tested, except for the third-line treatments for HCV genotype 1 interferon eligible and HCV genotype 3 interferon ineligible patients (see Data S1 page 63 for detailed results). Scenarios which examined less severe health and cost consequences of remaining infected resulted in the recommendation of two rather than three lines of sequential therapy for HCV genotype 3 interferon-ineligible patients. When the cost of treatment at cirrhosis was increased the cost-effective third-line strategy changed from SOF + PR to SOF + DCV for HCV genotype 1 interferon-eligible patients.
Conclusions
Main Findings
Patients at the F3 stage can be effectively and cost-effectively treated using available regimens in a sequential fashion to avoid progression to cirrhosis. The cost-effective strategies are those with at least two lines of treatment which ensures that a minimal number of patients progress to cirrhosis. These results are robust to a series of scenario analyses and are consistent with clinical guidelines which recommend that patients should be treated to avoid progression to cirrhosis.[30,41]
Peginterferon still has a role to play in the management of chronic hepatitis C, namely in HCV genotypes 2, 3 and 4 treatment-naïve patients. In these patients, the new DAAs should be the second line of treatment for patients who fail to achieve SVR with PR or those who are intolerant to interferon-based therapy. In HCV genotype 1, SOF + LED over 8 weeks is the cost-effective first line of treatment and 3D ± RBV is the cost-effective second line of treatment.
There is some uncertainty regarding both whether a third line of treatment should be offered and, if so, what this should be. By this point, the most cost-effective treatment options have generally been utilised and the remaining options offer only marginal benefits. Furthermore, there is additional uncertainty in the SVR rates of treatments as the third line. The data used in the model were obtained from studies including patients who had generally received only one prior treatment line as data specific to third-line patients were not available. Despite this uncertainty, the value of conducting further research to identify the cost-effective third line of treatment is likely to be low, as few patients reach this point in the treatment pathway.
The cost-effective strategies are shown at a cost-effectiveness threshold of £20 000 per QALY. Their ICERs indicate the magnitude of their cost-effectiveness and the impact of using lower cost-effectiveness thresholds. In treatment-naïve interferon-eligible patients, a cost-effectiveness threshold smaller than £5000 per QALY gained would not change the cost-effective strategy. The results are more sensitive in interferon-ineligible patients: the cost-effective strategies remain unchanged unless the cost-effectiveness threshold is smaller than £12 000 per QALY.
Comparison With Other Studies
A number of cost-effectiveness studies on the new DAAs have been published in the past couple of years, in unison with their market entry.[12–17,19,42–48] The studies typically compare each of the new DAAs against the current standard of care, boceprevir or telaprevir in HCV genotype 1 and PR in HCV genotype 2 and 3. No study has included all the currently available DAAs nor allowed for retreatment explicitly, with the exception of Zhao et al. in a retreatment scenario.[44] Although some studies analyse treatment-experienced patients separately,[12,14,15,19] this does not allow a comparison of optimal use of DAAs at different positions in the treatment pathway (e.g. first- vs. second-line use).
It is difficult to compare the findings of studies set in different countries due to the different parameter inputs, particularly drug prices. Generally, SOF + LED was found to be cost-effective for HCV genotype 1.[12,16,19] Some studies comparing 3D ± RBV and SOF + LED for HCV genotype 1 found 3D ± RBV to be cost-effective but its price was lower than SOF + LED.[12,16,19,43,44,48] Previous studies in HCV genotypes 2 and 3[12,14,16,42,47] suggest that, in line with the present study, SOF-based regimens are not cost-effective first-line treatments under conventional thresholds used in the UK. Similar results were found in HCV genotype 4,[12,42] although treatments were evaluated to a limited extent in this subgroup.
Strengths and Limitations
This paper reports the first study directly comparing the cost-effectiveness of all new DAAs simultaneously. The model structure reflects clinical practice in that patients who fail the first treatment can be retreated. In addition, it allows for treatment at the cirrhosis and decompensated cirrhosis stage, as per NHS current practice. The thoroughness of the NICE appraisal process ensures that all available evidence on the new DAAs has been considered and adequately scrutinised. Sensitivity analyses explored the robustness of the results to key uncertainties and provide added confidence in the conclusions.
The model does not include the possibility of reinfection and onward transmission to uninfected individuals. Excluding reinfection is likely to overestimate the benefits and underestimate the costs of more effective treatments since re-infected patients require new treatment. Conversely, excluding new infections may underestimate the benefits of more effective treatments. This limitation is likely to have a small impact on the results given the low risk of reinfection and onward transmission of the F3 patient population. Patients at the F3 stage are older;[49] older injecting drug users are less likely to share needs than young drug users, and therefore at a lower risk of reinfection and onward transmission.[50]
Remaining Areas of Uncertainty
The cost-effective strategies are subject to some uncertainty due to the limited clinical evidence. Some SVR rates were generalised between patient subgroups: (i) from noncirrhotic to F3 patients, (ii) from treatment-experienced to PR to treatment-experienced to the new DAAs, (iii) from treatment-experienced to one treatment line to treatment-experienced to two treatment lines, (iv) from HCV genotype 1 to HCV genotype 4, and (v) from interferon-eligible to interferon-ineligible. This was also conducted for the marketing authorisations of the new DAAs as well as reimbursement decisions made by bodies such as NICE. The impact on the results is difficult to predict. It depends on the extent to which these generalisations of evidence may have under- or over-estimated the differences in SVR rates between treatments.
There is also uncertainty regarding how the profile of patients coming forward for treatment may change in the future (i.e. disease stage, HCV genotype, co-morbidities). Historically, approximately 5000 people presented for treatment per year in England.[51] However, there are a large number of patients who are undiagnosed or not engaging with the health care system, who may be eligible for treatment.[52] These patients are typically in harder to reach populations and may have difficulty in adhering to treatment. This can have implications to the SVR rates and costs, as well as the likelihood of re-infection and onward transmission, which in turn may impact on the cost-effectiveness results.
Areas for Future Research
Questions remain on the cost-effective treatment strategy in patients with milder liver disease (METAVIR stage F0–F2). These patients differ from those at F3 for a number of reasons. Firstly, patients with milder liver disease may have better response rates than the general noncirrhotic population, on which most of the SVR rates used in the model are based. Secondly, these patients have a slower rate of progression to cirrhosis. They therefore benefit less from avoiding the development of cirrhosis. For these reasons, more research is required on the cost-effective treatment strategy in patients with milder liver disease. This research should explore the heterogeneous nature of the population with milder liver disease as this includes a significant proportion of individuals who inject drugs and/or are in hard to reach populations. These subgroups have important features relating to the ways in which they access care, their likelihood of transmitting infection to others, and their likelihood of reinfection following successful treatment, which should be reflected in any future cost-effectiveness analysis.
Implications for Policy
Patients at the METAVIR F3 stage should be treated intensively to avoid progression to cirrhosis. Treatment should be sequential and comprise at least two therapy lines. In HCV genotype 1, treatment-naïve, SOF + LED over 8 weeks is the cost-effective first line option. In all other treatment-naïve, interferon-eligible patients, PR should be used as the first line. The new DAAs should be used as the second and third line of treatments. Given the large eligible population, the costs of implementing a cost-ineffective treatment strategy are high, with potentially adverse impacts on the health of other patients. This study shows that cost-effectiveness analysis can be used to inform the cost-effective treatment strategy for each HCV genotype in a way that reflects the full treatment pathway. Regional price negotiation can be incorporated using the price tool, available to download from the Supporting information (Data S2 http://onlinelibrary.wiley.com/wol1/doi/10.1111/apt.13775/suppinfo#). This information is of significant benefit to health care providers with budget limitations and provides a sound scientific basis for drug treatment choices.